EVALUATION
Cloze Activity
Case Study
The extraction of Iron from its ores takes place in the blast furnace. Below is a simplified diagram of a blast furnace. Study it and answer the questions that follow.
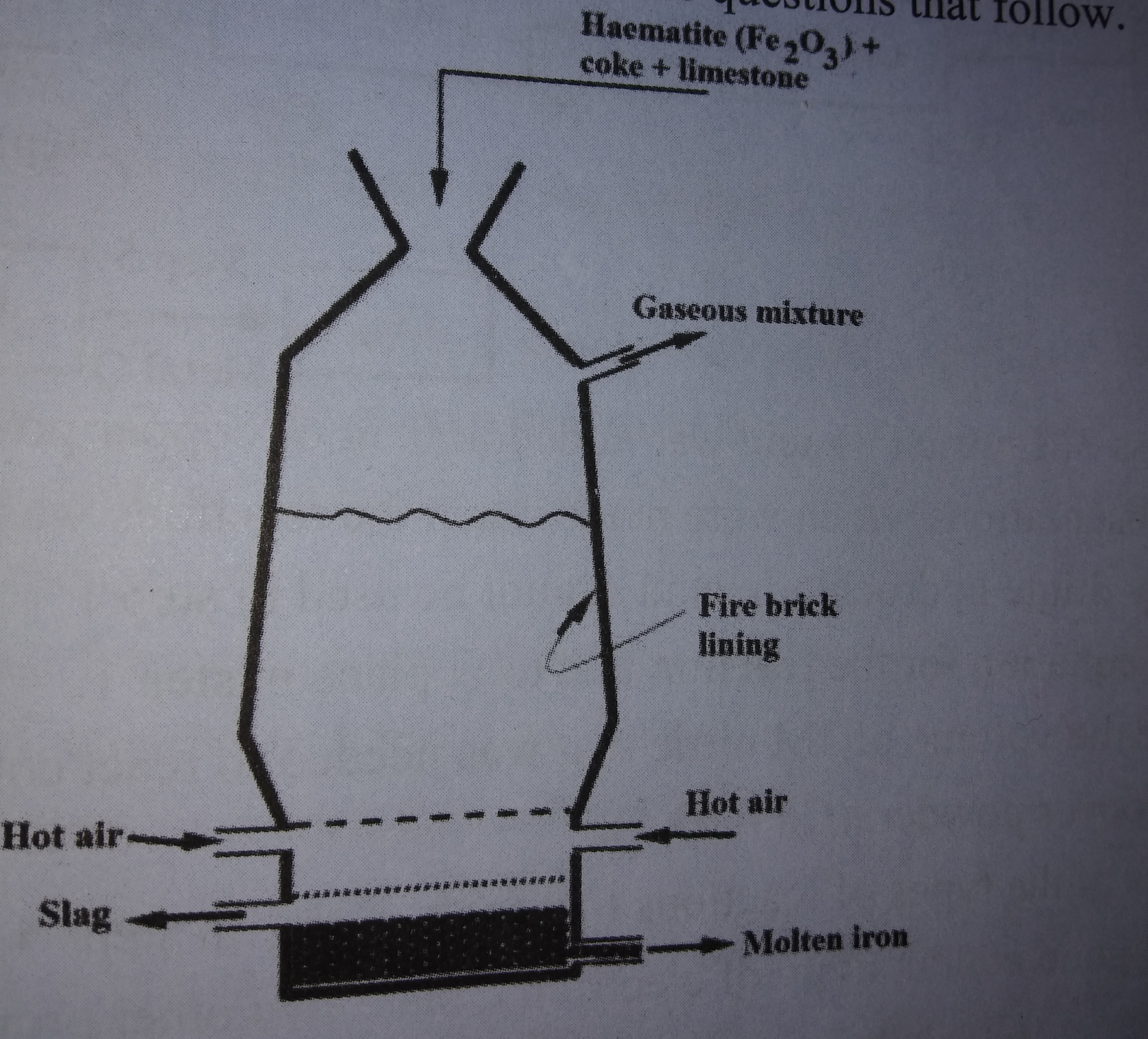 |
a) Name:
i) One of the substances in the slag. (1mk)
ii) Another iron ore material used in the blast furnace. (1mk)
iii) One gas which is recycled. (1mk)
b) Describe the processes which lead to the formation of Iron in the blast furnace. (3mks)
c) State the purpose of limestone in the blast furnace. (1mk)
d) Give a reason why the melting point of iron obtained from the blast furnace is 1200 degrees celcious while that of pure iron is 1535 degrees celcious. (2mks)
e) State two uses of steel. (2mks)
Licensed under the Creative Commons Attribution Share Alike License 4.0