A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
Refers to a structure in which covalent bonds holds atoms together to form molecules and the resultant molecules are held together by intermolecular forces. Substance with molecular structures are usually gases or liquids at room temperature.
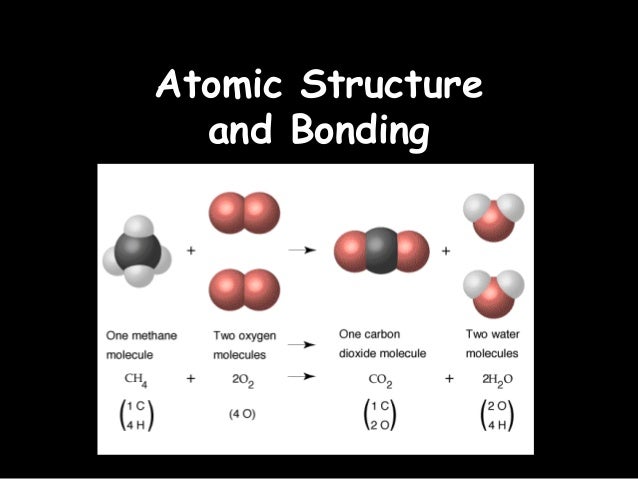
A chemical bond is formed when atoms of the same or different elements share, gain, donate or delocalize their outer
electrons to combine during chemical reactions inorder to be stable.
Atoms have equal number of negatively charged electrons in the energy levels and positively charged protons in the nucleus.
Atoms are chemically stable if they have filled outer energy level.
An energy level is full if it has duplet (2) or octet (8) state in outer energy level.