DATIVE BOND
Dative /coordinate bond
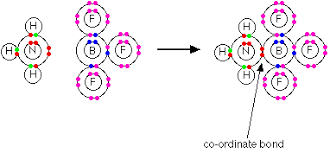
A dative/coordinate bond is a covalent bond formed when a lone pair of electrons is donated then shared to an electron-deficient species/ion/atom.
During dative/coordinate bonding, all the shared pair of electrons are donated by one of the combining/bonding species/ ion/atom.
Like covalent bonding, coordinate /dative bond is mainly formed by non-metals.
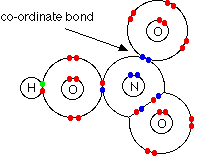
DRAW THE DATIVE BOND ON H3O+