COVALENT BOND
COVALENT BOND
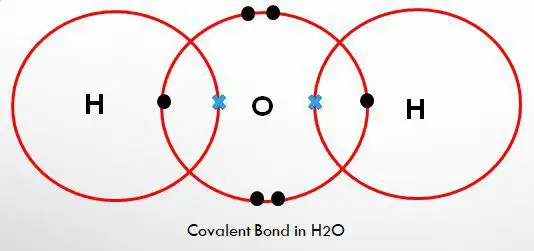
A covalent bond is formed when atoms of the same or different element share some or all the outer energy level electrons to combine during chemical reactions inorder to attain stable duplet or octet.
A shared pair of electrons is attracted by the nucleus (protons) of the two atoms sharing.
Covalent bonds are mainly formed by non-metals to form molecules.
A molecule is a group of atoms of the same or different elements held together by a covalent bond.
The number of atoms making a molecule is called atomicity.
Noble gases are monatomic because they are stable and thus do not bond with each other or other atoms.
Most other common gases are diatomic.
The more the number of electrons shared, the stronger the covalent bond.
A pair of electrons that do not take part in the formation of a covalent bond is called a lone pair of electrons.
Note:
After bonding the following intramolecular forces exist:
(i)the attraction of the shared electrons by both nucleus /protons of the atoms
(ii) the repulsion of the nucleus of one atom on the other.
(iii)balance of the attraction and repulsion is maintained inside/intramolecular/within the molecule.
EXAMPLES



Assignment: Draw the structure of NH4+