PROPERTIES OF HYDROGEN GAS
strong>Properties of Hydrogen gas
(a)Physical properties
- Hydrogen is a neutral ,colourless and odourless gas.
When mixed with air it has a characteristic pungent choking smell
- It is insoluble in water thus can be collected over water.
- It is the lightest known gas.
It can be transferred by inverting one gas jar over another.
(b)Chemical properties.
(i)Burning
- Hydrogen does not support burning/combustion.
When a burning splint is inserted into a gas jar containing Hydrogen, the flame is extinguished /put off Pure dry hydrogen burn with a blue quiet flame to form water.
When a stream of pure dry hydrogen is ignited, it catches fire and continues to burn with a blue flame.
III. Impure (air mixed with) hydrogen burns with an explosion.
Small amount/ volume of air mixed with hydrogen in a test tube produce a small explosion as a “pop” sound.
This is the confirmatory test for the presence of Hydrogen gas. A gas that burns with a “pop” sound is confirmed to be Hydrogen.
2. When a stream of dry hydrogen gas is passed through black copper (II) oxide, hydrogen gas gains the oxygen from copper(II)oxide.
Black copper (II) oxide is reduced to brown copper metal.
Black copper(II)oxide is thus the Oxidizing agent.
Hydrogen gas is oxidized to Water. Hydrogen is the Reducing agent.
Water as an Oxide of Hydrogen
Burning is a reaction of an element with Oxygen.
The substance formed when an element burn in air is the oxide of the element.
When hydrogen burns, it reacts/ combines with Oxygen to form the oxide of Hydrogen.
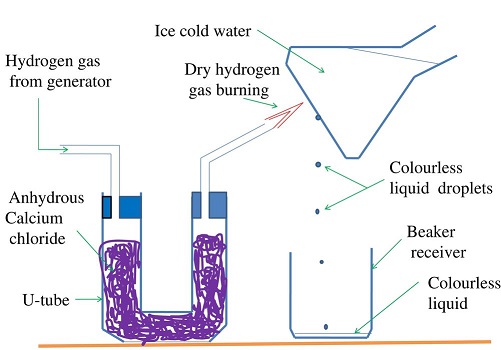
The oxide of Hydrogen is called water. Hydrogen is first dried because a mixture of Hydrogen and air explode.
The gas is then ignited .
The products condense on a cold surface/flask containing a freezing mixture.
A freezing mixture is a mixture of water and ice.
1. What are the products of burning hydrogen gas?
2. Write the equation of reaction of hot iron (II) oxide with hydrogen gas
3. state an experiment of proving that water is an oxide of hydrogen