PRPARATION OF HYDROGEN GAS
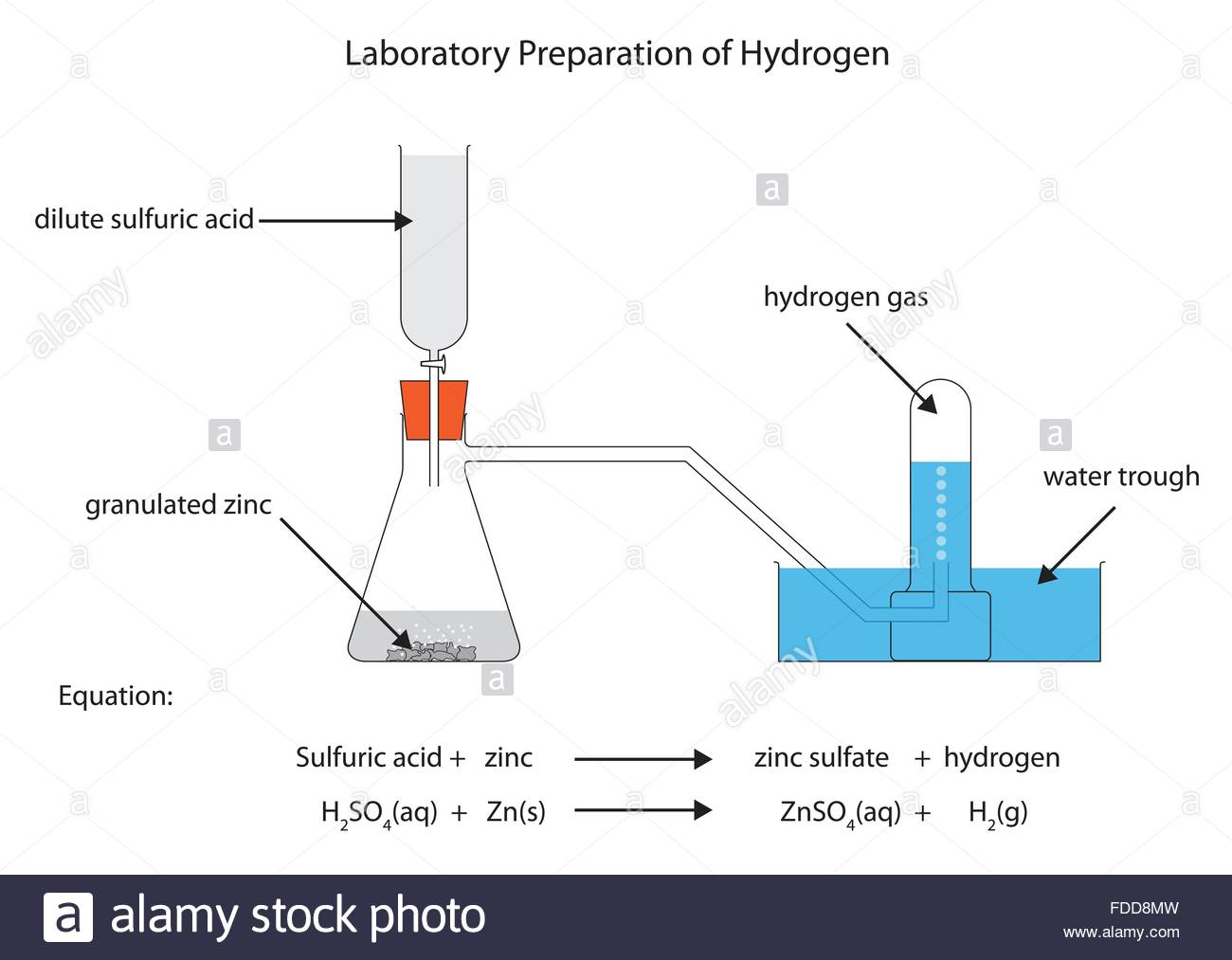
Observation/Explanation
Zinc reacts with dilute sulphuric(VI)/hydrochloric acid to form a salt and produce hydrogen gas.
When the acid comes into contact with the metal, there is rapid effervescence/ bubbles /fizzing are produced and a colourless gas is produced that is collected:
(i) over water because it is insoluble in water
(ii)through downward displacement of air/upward delivery because it is less dense than air.
The first gas jar is impure.
It contains air that was present in the apparatus.
Copper(II)sulphate(VI)solution act as catalyst.
1. What is the colour of Hydrogen gas
2. Why is the gas collected by over water method
3. Lead metal and sulphuric acid is not used in preparation of hydrogen gas. Explain
4. Nitric acid is not used in preparation of hydrogen gas. Explain