From the graph, the volume of the gas is proportional to the temperature in Kelvins.
Therefore, Charles Law states that:
The volume of a fixed mass of a gas is directly proportional to its absolute temperature provided the pressure is kept constant.
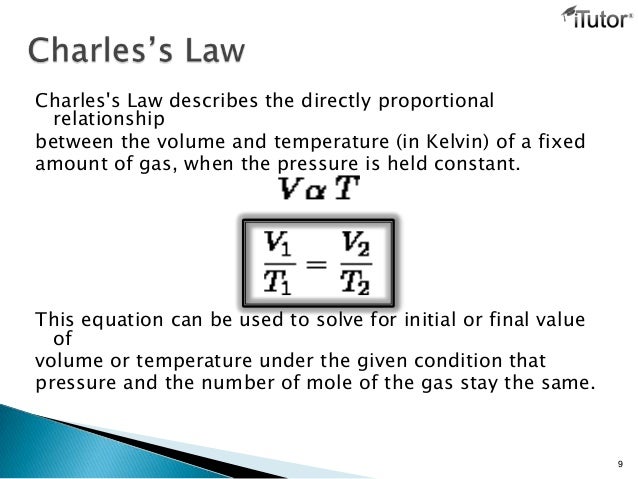
From the graph, the volume of the gas is proportional to the temperature in Kelvins.
Therefore, Charles Law states that:
The volume of a fixed mass of a gas is directly proportional to its absolute temperature provided the pressure is kept constant.

Licensed under the Creative Commons Attribution Share Alike License 4.0