At 4°C, water has Maximum density.
Normally, liquids contract on cooling & the density increases. However, water is special. It contracts when cooled, down to a temperature of 4°C (normal behaviour).Water attains its maximum density at 4°C . On further cooling , it begins to expand ( instead of contracting) and thus less dense, until it reaches 0°C and turns into ice. Solid water or ice is thus less denser than liquid water and so ice floats on water.
The following is a video to explain how this happens. You can replay the video or pause as you wish until you understand the concept.
Density =Mass/Volume
.
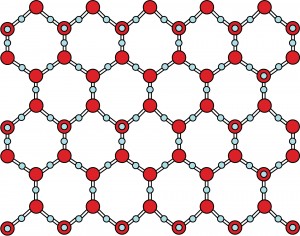 Structure of ice ( solid water)
Structure of ice ( solid water)