Aluminium ORES
- Bauxite; AlOx(OH)3-2x
- Kaolinite (a form of clay); [Al2 (OH)4 Si2O5]
Aluminium is above carbon in the series so it cannot be extracted from its ores in the same way as carbon.
Electrolysis of molten Aluminium ore (alumina) must be used.
As energy is required to melt the alumina and electrolyse it, a large amount of energy is required.
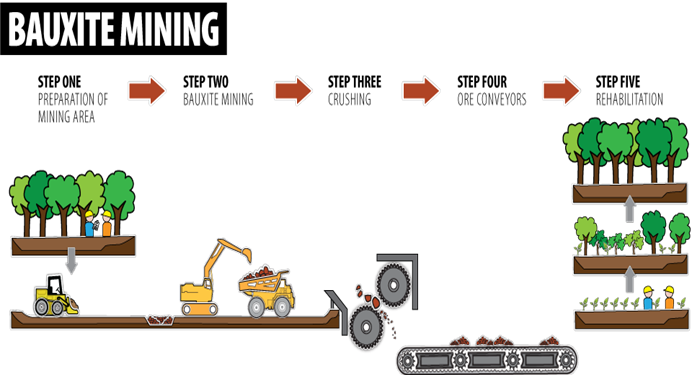
BAUXITE Aluminium ore
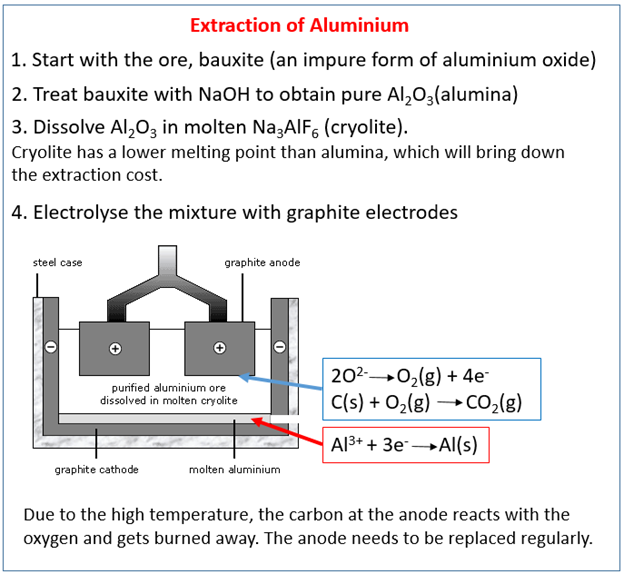
Bauxite contains alumina (Al2O3 Aluminium oxide) plus impurities such as iron oxide – it is purified before use.
CRYOLITE
Aluminium oxide has a very high melting point.
Adding cryolite lowers the melting point and saves energy.
Unlike iron, Aluminium cannot be extracted using carbon
(Aluminium is above carbon in the reactivity series).
Reactive metals are extracted using electrolysis.
Electrolysis is expensive, it requires a lot of energy because; the ore must be molten have high melting points and electricity is needed for the electrolysis process.
Solid ionic compounds do not conduct electricity.
This is because the ions are not free to move and can only do that by;
Dissolving in water or melting which allows.
The ions to move freely.
Positive ions move to the negative electrode.
Negative ions move to the positive electrode.