Activity
Study the image above and do the activity below.
On yout notebook categorize the metals in the reactivity series with the method of extraction shown in the flow chart either through electrolysis, reduction or roasting.
OCCURRENCE
THEORY
The method used to extract metals depends on the :
REACTIVITY SERIES
K Na Ca Mg Al C Zn Fe H Cu Ag
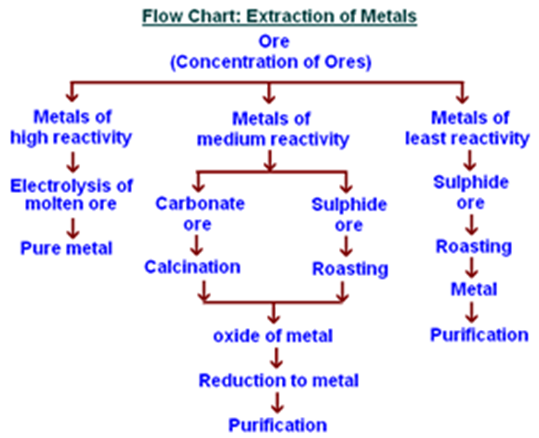
Study the image above and do the activity below.
On yout notebook categorize the metals in the reactivity series with the method of extraction shown in the flow chart either through electrolysis, reduction or roasting.
Licensed under the Creative Commons Attribution Share Alike License 4.0