CHEMICAL PROPERTIES OF ALKANES
Chemical properties
(i)Burning.
Alkanes burn with a blue/non-luminous non-sooty/non-smoky flame in excess air to form carbon(IV) oxide and water.
Alkane + Air -> carbon(IV) oxide +water (excess air/oxygen)
Alkanes burn with a blue/non-luminous no-sooty/non-smoky flame in limited air to form carbon(II) oxide and water.
Alkane + Air -> carbon(II) oxide +water (limited air)
Examples
1.(a) Methane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Methane + Air -> carbon(IV) oxide +water (excess air/oxygen)
CH4(g) + 2O2(g)-> CO2(g) +2H2O(l/g)
(b) Methane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Methane + Air -> carbon(II) oxide +water (excess air/oxygen)
2CH4(g)+ 3O2(g)-> 2CO(g) +4H2O(l/g)
2.(a) Ethane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Ethane + Air -> carbon(IV) oxide +water (excess air/oxygen)
2C2H6(g)+ 7O2(g)-> 4CO2(g) +6H2O(l/g)
(b) Ethane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Ethane + Air -> carbon(II) oxide +water (excess air/oxygen)
2C2H6(g)+ 5O2(g)-> 4CO(g) +6H2O(l/g)
3.(a) Propane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Propane + Air -> carbon(IV) oxide +water (excess air/oxygen)
C3H8(g)+ 5O2(g)-> 3CO2(g) +4H2O(l/g)
(b) Ethane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Propane + Air -> carbon(II) oxide +water (excess air/oxygen)
2C3H8(g)+ 7O2(g)-> 6CO(g) +8H2O(l/g)
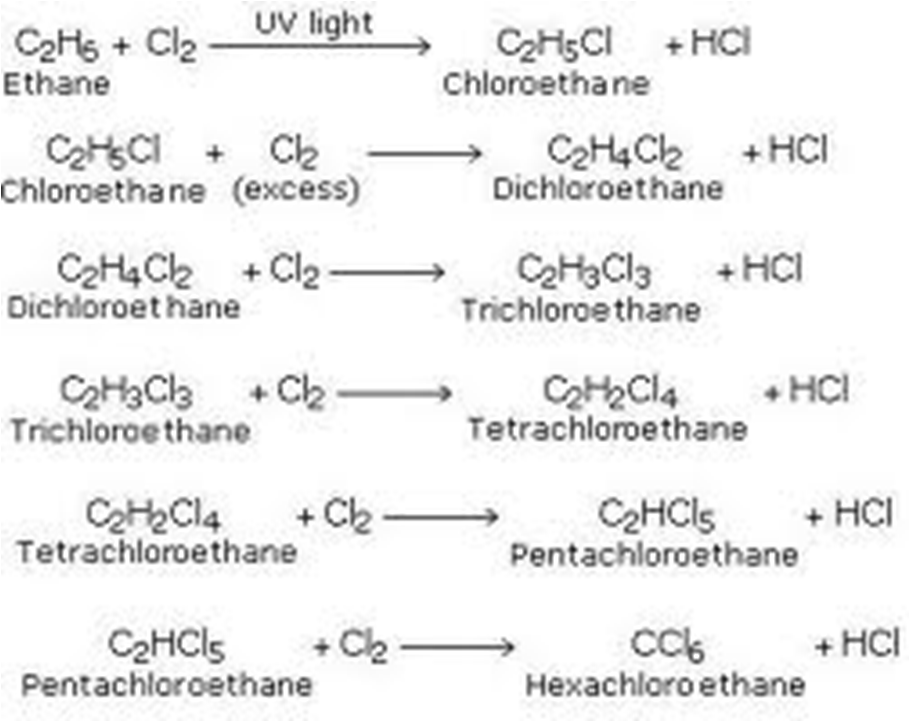
ii)Substitution
Substitution reaction is one in which a hydrogen atom is replaced by a halogen in presence of ultraviolet light.
Alkanes react with halogens in presence of ultraviolet light to form halogenoalkanes.
Uses of alkanes
1.Most alkanes are used as fuel e.g.
Methane is used as biogas in homes.
Butane is used as the Laboratory gas.
2.On cracking ,alkanes are a major source of Hydrogen for the manufacture of ammonia/Haber process.
3.In manufacture of Carbon black which is a component in printers ink.
4.In manufacture of useful industrial chemicals like methanol, methanol, and chloromethane.