LABORATORY PREPARATION OF ALKANES
School laboratory preparation of alkanes
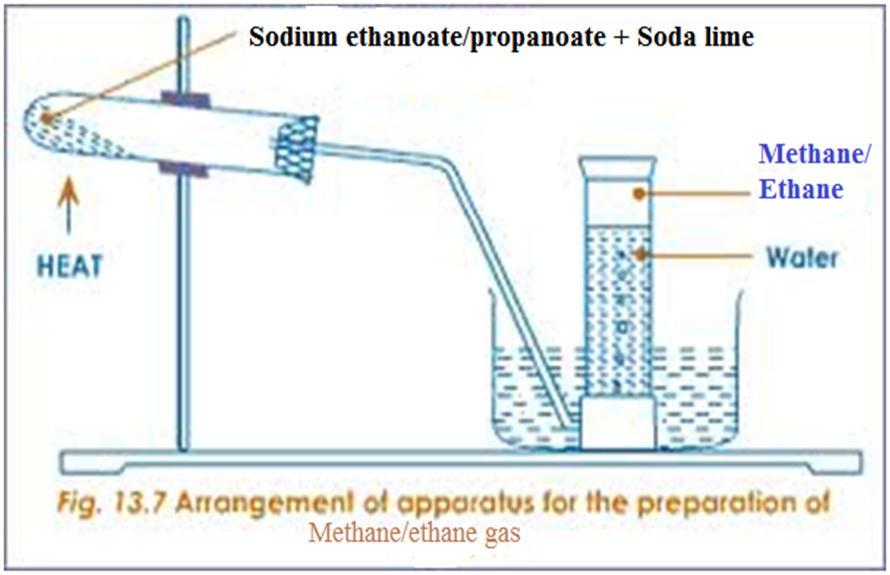
In a school laboratory, alkanes may be prepared from the reaction of a sodium alkanoate with solid sodium hydroxide /soda lime.
Chemical equation:
Sodium alkanoate+soda lime -> alkane +Sodium carbonate
C2 H2n+2 COONa(s) +NaOH(s) -> C2 H2n+2 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CnH2n+1 in CnH2n+1COONa(s) to form C2 H2n+2.
Examples
- Methane is prepared from the heating of a mixture of sodium ethanoate and soda lime and collecting over water
-
Sodium ethanoate + soda lime->
methane + Sodium carbonate
CH3COONa(s) +NaOH(s) ->C H4 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3 in CH3COONa(s) to form CH4.
- Ethane is prepared from the heating of a mixture of sodium propanoate and soda lime and collecting over water
Sodium propanoate + soda lime->
ethane+Sodium carbonate
CH3 CH2COONa(s) +NaOH(s)->CH3 CH3 +Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3 CH2 in CH3 CH2COONa (s) to form CH3 CH3
write the balanced chemical equation for the above reaction