PHYSICAL PROPERTIES OF HCl
Dry hydrogen chloride is a molecular compound that is joined by covalent bonds between the atoms.
- It has no effect on dry litmus papers. The gas is polar thus dissolves in water and ionize completely to free H+ that are responsible for turning wet/damp/moist blue litmus paper red.
- When dissolved in non-polar solvent, it does not dissociate / ionize to H+ that changes the colour of litmus solution. When dissolved in polar solvent like water, it dissociate ionize to H+ that changes litmus solution to red.
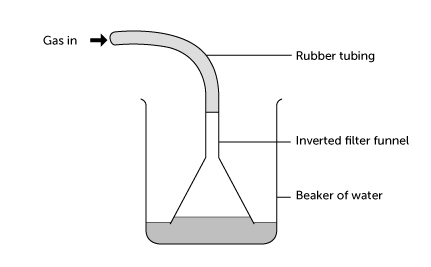
- The gas is higly soluble in water. It therefore forms a fountain

1. What property of HCl is demonstrated by the fountain experiment?
2. Why is it that HCl has no effect on dry litmus papers?
3. What is the colour of HCl gas?
4. What is the test of HCl gas?