Read the following information on Ionic bonding
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions.
In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
Ions: atoms that have a charge due to gain or loss of electrons.
–Anion: (-) charged atom.
–Cation: (+) charged atom.
Examples of substances with Ionic Bonds:
1. Sodium chloride - NaCl - table salt
2. Calcium chloride - CaCl2
3. Sodium hydroxide - NaOH
4. Sodium fluoride - NaF – fluoride in toothpaste
5. Iron (III) Oxide – Fe2 O3 - rust
6. Calcium hydroxide – Ca(OH)2
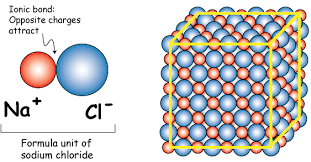
Sodium has 1 electron in its outermost shell, and chlorine has 7 electrons. It is easiest for sodium to lose its electron and form a +1 ion, and for chlorine to gain an electron, forming a -1 ion. If sodium can transfer it's "spare" electron to chlorine (as shown above), both atoms will satisfy their full outer shell requirements, and an ionic bond will be formed. If large groups of sodium and chlorine atoms bond this way, the result is a three-dimensional structure with alternating sodium and chlorine ions:
What are the main properties of ionic compounds?
They form crystals. ... They have higher enthalpies of fusion and vaporization than molecular compounds. They are hard. They are brittle. They have high melting points and also high boiling points. They conduct electricity but only when they are dissolved in water.

They form crystals. ... They have higher enthalpies of fusion and vaporization than molecular compounds. They are hard. They are brittle. They have high melting points and also high boiling points. They conduct electricity but only when they are dissolved in water.