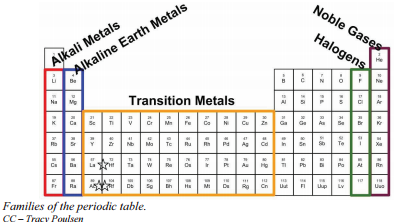
Elements in the same group are said to belong to the same chemical family. Thus a chemical family refers to a group of elements with the same number of valence electrons and hence in the same group of the periodic table.
Families of the Periodic Table
Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. A group is a vertical column of the periodic table. All of the 1A elements have one valence electron. This is what causes these elements to react in the same ways as the other members of the family. The elements in 1A are all very reactive and form compounds in the same ratios with similar properties with other elements. Because of their similarities in their chemical properties, Mendeleev put these elements into the same group. Group 1A is also known as the alkali metals. Although most metals tend to be very hard, these metals are actually soft and can be easily cut.
Group 2A is also called the alkaline earth metals. Once again, because of their similarities in electron configurations, these elements have similar properties to each other. The same pattern is true of other groups on the periodic table. Remember, Mendeleev arranged the table so that elements with the most similar properties were in the same group on the periodic table.
It is important to recognize a couple of other important groups on the periodic table by their group name. Group 7A (or 17) elements are also called halogens. This group contains very reactive nonmetal elements.
The noble gases are in group 8A. These elements also have similar properties to each other, the most significant property being that they are extremely unreactive, rarely forming compounds. We will learn the reason for this later, when we discuss how compounds form. The elements in this group are also gases at room temperature.