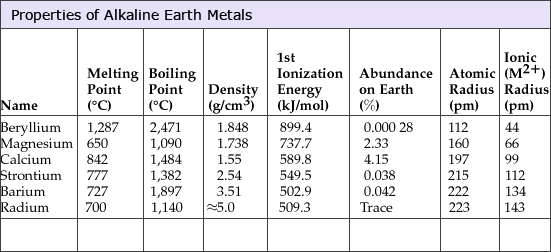
PROPERTIES OF ALKALINE EARTH METALS
Properties of Alkaline Earth Metals
The alkaline earth metals (beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)) are a group of chemical elements in the s-block of the periodic table with very similar properties:
- shiny
- silvery-white
- somewhat reactive metals at standard temperature and pressure
- readily lose their two outermost electrons to form cations with a 2+ charge
- low densities
- low melting points
- low boiling points
The alkaline earth metals comprise the group 2 elements. All the discovered alkaline earth metals occur in nature.
Reactions of Alkaline Earth Metals
All the alkaline earth metals have two electrons in their valence shell, so they lose two electrons to form cations with a 2+ charge. Most of the chemistry has been observed only for the first five members of the group; the chemistry of radium is not well established due to its radioactivity.
In chemical terms, all of the alkaline metals react with the halogens to form ionic alkaline earth metal halides. All the alkaline earth metals except beryllium also react with water to form strongly alkaline hydroxides which should be handled with great care. The heavier alkaline earth metals react more vigorously than the lighter ones.
The alkaline metals have the second-lowest first ionization energies in their respective periods of the periodic table. This is due to their low effective nuclear charges and the ability to attain a full outer shell configuration by losing just two electrons. The second ionization energy of all of the alkaline metals is also somewhat low.
Beryllium is an exception. It does not react with water or steam, and its halides are covalent. All compounds that include beryllium have a covalent bond. Even beryllium fluoride, which is the most ionic beryllium compound, has a low melting point and a low electrical conductivity when melted.
SUMMARY OF THE PHYSICAL PROPERTIES
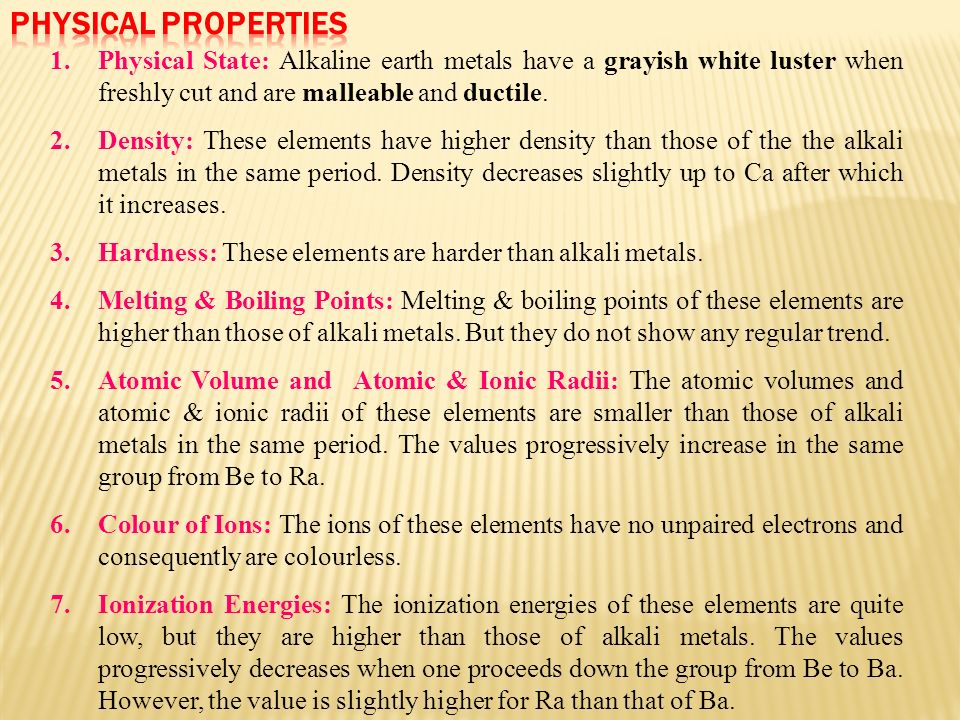
STATE SOME PHYSICAL PROPERTIES OF AALKALINE EARTH METALS