Crystallization
-
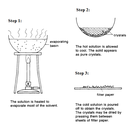
Crystallisation: Used to separate a dissolved heat-liable (will decompose upon heating and hence can sublime) solid (solute) from a solution.
You will need a saturated solution to being with. A saturated solution is a solution that contains the maximum amount of solute dissolved in a given volume of solvent at a particular temperature. Do not mix this up with a concentrated solution, which is a solution that contains lots of solute dissolved in it. The amount of solute in a concentrated solution may/may not be the maximum amount which can be dissolved in the solution.
First, you will need to heat to evaporate off most of the solvent from a solution to make a hot and nearly saturated solution. Else, if you already have a saturated solution, heat it up slightly such that the solution becomes hot.
After which, allow the hot solution to coll naturally. The solubility of the solute decreases as the solution is cooled, and the excess solute which can no longer be dissolved in the saturated solution crystallizes out of the solution. The crystals which are formed can be separated from the remaining solution by filtration.