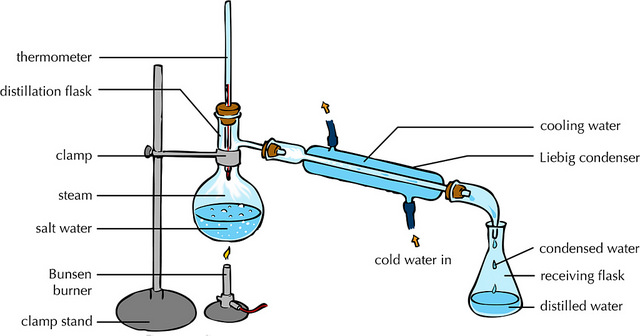
Fractional distillation involves:
(i)Heating the mixture in a conical/round bottomed /flat bottomed flask.
The pure substance with a lower boiling point and thus more volatile evaporates/boils/vapourizes first.
e.g.
Pure ethanol has a boiling point of 78oC.
Pure water has a boiling point of 100 oC at sea level/one atmosphere pressure.
When a miscible mixture of ethanol and water is heated, ethanol vapourizes /boils/ evaporates first because it is more volatile.