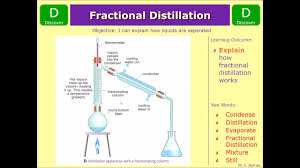
FRACTIONAL DISTILATION
(ii)The conical/round bottomed /flat bottomed flask is connected to a long glass tube called fractionating column.
The purpose of the fractionating column is to offer area of condensation for the less volatile pure mixture.
The fractionating column is packed with glass beads/broken glass/ porcelain/ shelves to increase the surface area of condensation of the less volatile pure mixture.
(iii)When the vapour rises it condenses on the glass beads/broken glass /porcelain / shelves which become hot.
When the temperature of the glass beads/broken glass/porcelain/shelves is beyond the boiling point of the less volatile pure substance, the pure substance rise and condensation take place on the glass beads/broken glass/porcelain/shelves at a higher level on the fractionating column.
The less volatile pure substance trickles/drips back down the fractionating column or back into the conical/round bottomed /flat bottomed flask to be heated again. e.g.
If the temperature on glass beads/broken glass/porcelain/shelves is beyond 78oC, the more volatile pure ethanol rise to condense on the glass beads/broken glass /porcelain /shelves higher in the fractionating column.
Water condenses and then drip/trickle to the glass beads /broken glass /porcelain /shelves lower in the fractionating column because it is less volatile.
iv)The fractionating column is connected to a liebig condenser.
The liebig condenser has a cold water inlet and outlet circulation.
The more volatile mixture that reach the top of the fractionating column is condensed by the liebig condenser into a receiver.
It is collected as the first fraction.
(v)At the top of the fractionating column, a thermometer is placed to note/monitor the temperature of the boiling mixtures
Pure substances have constant/fixed boiling point.
When one mixture is completely separated, the thermometer reading rises.
e.g. The thermometer reading remains at78oC when ethanol is being separated
When no more ethanol is being separated, the mercury/ alcohol level in the thermometer rises.
(vi)The second /subsequent fractions are collected in the receiver after noting a rise the mercury/alcohol level in the thermometer.
e.g.
The thermometer reading rises to 100oC when water is being separated.
It is passed through the liebig condenser with the cold water inlet and outlet circulation.
It is collected different receiver as the second/subsequent fraction.
vii)Each fraction collected should be confirmed from known physical/chemical properties/characteristic.
e.g.
Ethanol
Ethanol is a colourless liquid that has a characteristic smell . When it is put in a watch glass then ignited, it catches fire and burn with a blue flame.
Water
Water is a colourless liquid that has no smell/odour .
When it is put in a watch glass then ignited, it does not catch fire.