FORM 4 CHEMISTRY
Activity 3
Reading Activity: Rules for assigning oxidation numbers
1.All elements are zero.
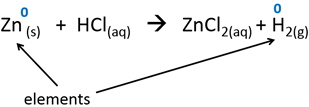
2.Charges of ions are their oxidation numbers.
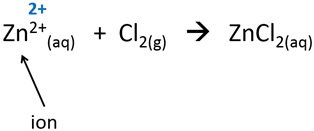
3.All compounds without a net charge are zero.
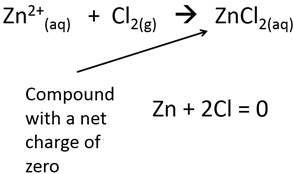
4.Hydrogen is almost always 1+ when found in a compound.
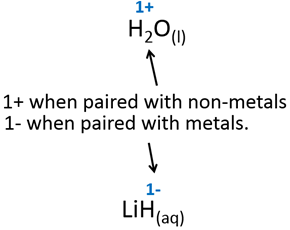
5.Oxygen is almost always 2- when found in a compound.
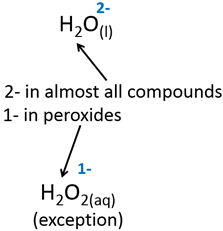
6.If a compound has a net charge, the sum of its atoms’ charges must equal the net charge.
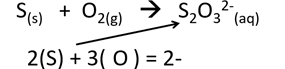
1. Calculate Oxidation Number of sulphur in 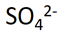
2. Calculate Oxidation Number of chlorine in ![]()
3. Calculate Oxidation Number of nitrogen in 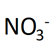
Licensed under the Creative Commons Attribution Share Alike License 4.0