PROPERTIES OF CARBON
Properties of Carbon
(i)Physical properties of carbon
Carbon occur widely and naturally as a black solid
It is insoluble in water but soluble in carbon disulphide and organic solvents.
It is a poor electrical and thermal conductor.
Chemical properties of carbon
Carbon burns in excess air/Oxygen with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas. Carbon burns in limited air with a blue non-sooty/non-smoky flame forming Carbon (II) oxide gas.
Carbon (IV) oxide gas dissolve in water to form a weak acidic solution of Carbonic (IV)acid.
C(s) + O2 (g) -> CO2 (g) (in excess air)
2C(s) + O2 (g) -> 2CO(g) (in limited air)
CO2 (g) + H2O (l) -> H2CO3 (aq) (very weak acid)
Carbon is a reducing agent.
For ages it has been used to reducing metal oxide ores to metal, itself oxidized to carbon(IV)oxide gas.
Carbon reduces black copper(II)oxide to brown copper metal
2CuO(s) + C(s) -> 2Cu(s) + CO2 (g)
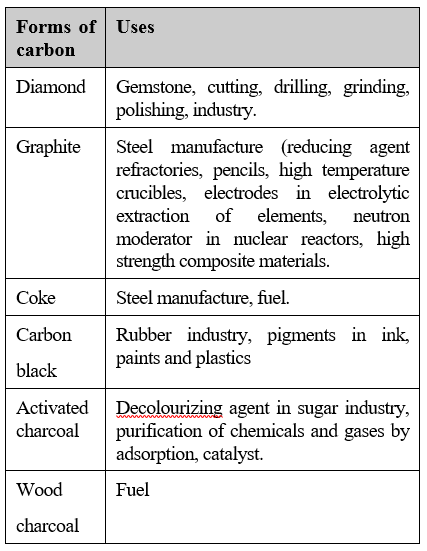
USES OF CARBON
- It makes up for 18% of the human body. Sugar, glucose, proteins etc are all made of it. ...
- Carbon in its diamond form is used in jewellery obviously. ...
- Amorphous carbon is used to make inks and paints. ...
- Graphite is used as the lead in your pencils. ...
- One of the most important uses is carbon dating
1. State the physical property of graphite that makes it a good conductor of electricity.
2. Diamond is the hardest known substance on the earths surface. Explain?