Reading Activity
2. What method of gas collection is used during the preparation of carbon (II) oxide.
Over water because the gas is insoluble in water.
Downward delivery because the gas is 1 ½ times denser than air .
3. What is the purpose of :
(i) Potassium/sodium hydroxide in Method 1
To absorb/ remove carbon (II) oxide produced during the reaction.
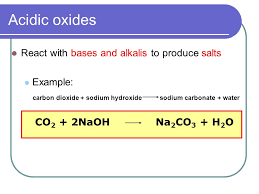
2KOH + CO2 = K2 CO3 + H2 O
(ii) Concentrated sulphuric(VI)acid in Method 2 Dehydrating agent –removes the element of water (Hydrogen and Oxygen in ratio 2:1) present in both methanoic and ethan-1,2-dioic acid.
- Describe the smell of carbon(II)oxide.
Colourless and odourless.
- State and explain the observation made when carbon(II)oxide is bubbled in lime water for a long time.
No white precipitate is formed.
- Dry and wet/moist/damp litmus papers were separately put in a gas jar containing dry carbon(II)oxide gas.
State and explain the observations made.
Observation
-blue dry litmus paper remains blue
-red dry litmus paper remains red
- wet/moist/damp blue litmus paper remains blue
- wet/moist/damp red litmus paper remains red
Explanation
Carbon(II)oxide gas is a molecular compound that does not dissociate /ionize to release H+ ions and thus has no effect on litmus papers.
Carbon(II)oxide gas is therefore a neutral gas.

State the observations made in flame K.
Gas burns with a blue flame

Carbon(II)oxide is a reducing agent. Explain